by Loïc
April 2003 - updated Dec 2006
 For a rather long time very plausible hypothesis were emitted. My comment needing to be short (an exam to pass soon !!) you are kindly requested to look at the pages that already describe this matter (for instance our first page!... !...) in order to get a general idea on this topic. For a rather long time very plausible hypothesis were emitted. My comment needing to be short (an exam to pass soon !!) you are kindly requested to look at the pages that already describe this matter (for instance our first page!... !...) in order to get a general idea on this topic.
 Nevertheless, we remind the scenario already evoked among others on The Pop-pop Page.
The pipe is full of water. It is heated. Water vaporizes. Steam forces liquid water to exhaust at the bottom of the pipe. Then a low pressure is created. (How? We will try to answer…). Some water is sucked in the pipe, and the cycle restarts. Nevertheless, we remind the scenario already evoked among others on The Pop-pop Page.
The pipe is full of water. It is heated. Water vaporizes. Steam forces liquid water to exhaust at the bottom of the pipe. Then a low pressure is created. (How? We will try to answer…). Some water is sucked in the pipe, and the cycle restarts.
 It makes sense, but to be sure of some things we would need… hey, a transparent coil… Hum, yes but… we are not glass workers… (November 2005: there is still much to learn, but you could see here how to make a glass coil). It makes sense, but to be sure of some things we would need… hey, a transparent coil… Hum, yes but… we are not glass workers… (November 2005: there is still much to learn, but you could see here how to make a glass coil).
 Time goes on. We have other things to do… And then, on a beautiful day, the solution is here just in front of us! It is hard to believe it… We think that there will be something wrong, cold water inlet will make the magnificent pipe coil that we just discovered brake in small pieces… But it has the right size, the right spiral number, in short: we were dreaming of it ! Time goes on. We have other things to do… And then, on a beautiful day, the solution is here just in front of us! It is hard to believe it… We think that there will be something wrong, cold water inlet will make the magnificent pipe coil that we just discovered brake in small pieces… But it has the right size, the right spiral number, in short: we were dreaming of it !
Were we talk about hardware :
 However, we have got only the coil. We miss a pipe of the same diameter… not easy to find, but a broken pipette and a welding torch will fit… Click of the piezoelectric lighter, flame adjustment… We turn the pipe while pulling on it… It is not perfectly straight, but it should fit… Pipes are bended as requested, and the trick is nearly finished… However, we have got only the coil. We miss a pipe of the same diameter… not easy to find, but a broken pipette and a welding torch will fit… Click of the piezoelectric lighter, flame adjustment… We turn the pipe while pulling on it… It is not perfectly straight, but it should fit… Pipes are bended as requested, and the trick is nearly finished…
 To connect these small pipes to the coil, pieces of silicone hose are used, and that's it. It is thought that the pipes ends touching each other inside the hose, this later shouldn't "breathe", furthermore it is temperature resistant. To connect these small pipes to the coil, pieces of silicone hose are used, and that's it. It is thought that the pipes ends touching each other inside the hose, this later shouldn't "breathe", furthermore it is temperature resistant.
 On one side: a pot, some water, some very old blue dye, a Depron board, and the pop-pop engine… On the other side: a movie camera on a pedestal, and let's go… On one side: a pot, some water, some very old blue dye, a Depron board, and the pop-pop engine… On the other side: a movie camera on a pedestal, and let's go…
 Hum no, miss a boiler… One, then two metallic caps, two strokes of pliers… a swab of cotton soaked with burning alcohol, and here we go… Hum no, miss a boiler… One, then two metallic caps, two strokes of pliers… a swab of cotton soaked with burning alcohol, and here we go…
Where action starts :
 Motor, action !... And… Matches are needed… Schrrrkkk Pcchhhh, this time heat is on ! Motor, action !... And… Matches are needed… Schrrrkkk Pcchhhh, this time heat is on !
 Due to the time needed for page loading, we are going to comment some pictures extracted from the video, but for those who wish you coulddownload the complete video sequences. Due to the time needed for page loading, we are going to comment some pictures extracted from the video, but for those who wish you coulddownload the complete video sequences.
 |
 |
 |
 |
|
Full of water (t 0) |
Vaporization and
drainage start (t 71s) |
Vaporization
continue (t 74s)
|
End of vaporization
and drainage (t 77s)
|
 |
 |
 |
 |
|
Empty (down to the water
level in the pot) (t 82s) |
Refilling (t 118s) |
Oscillating (t 129s) |
Oscillating... |
 It can be seen on the video that a small bubble is stuck
at the outlet of the farthest pipe. We could see, during other tests without this bubble, that it doesn't
influence the operation. In addition, we presume while waiting for complementary tests that it is the slight difference
(it is craftsmanship…!) in diameter between both pipes which is the cause of the small sucking of (non colored) water that occurs
in the nearest pipe at t=54 s. It can be seen on the video that a small bubble is stuck
at the outlet of the farthest pipe. We could see, during other tests without this bubble, that it doesn't
influence the operation. In addition, we presume while waiting for complementary tests that it is the slight difference
(it is craftsmanship…!) in diameter between both pipes which is the cause of the small sucking of (non colored) water that occurs
in the nearest pipe at t=54 s.
 Oscillation goes on untill the boiler is switched off. At that time, a slow but total water filling can be observed. Oscillation goes on untill the boiler is switched off. At that time, a slow but total water filling can be observed.
 The stroke is approximately 7 to 10 mm, the frequency being approximately 10 hz (It's hard to determine it with precision via the video,
due to the "long time of obturation under weak luminosity). The stroke is approximately 7 to 10 mm, the frequency being approximately 10 hz (It's hard to determine it with precision via the video,
due to the "long time of obturation under weak luminosity).
Where we start to think :
 First of all, we can see that the glass window located in
front of the camera is useless because the dilatations involved by the temperature changes do not make
the coil bursting out. First of all, we can see that the glass window located in
front of the camera is useless because the dilatations involved by the temperature changes do not make
the coil bursting out.
 Then, one thinks that science is something great ! Then, one thinks that science is something great !
 Finally, one verifies that the fore mentioned assumption was not so bad as it could have been.
Before going further, the test is repeated twice more… just to check that the result is the same. Finally, one verifies that the fore mentioned assumption was not so bad as it could have been.
Before going further, the test is repeated twice more… just to check that the result is the same.
 Therefore, it is obvious that the coil doesn't fill by itself, which is rather logic because its temperature must be something as 100°C… confirmed
by the instantaneous vaporization of a drop deposited on the pipe from which the flame has just been withdrawn. Therefore, it is obvious that the coil doesn't fill by itself, which is rather logic because its temperature must be something as 100°C… confirmed
by the instantaneous vaporization of a drop deposited on the pipe from which the flame has just been withdrawn.
 Once the oscillation initiated, it can be seen that there is a water stream inside the pot. This raises the question to know if this only
oscillation could be sufficient to propel a boat. Once the oscillation initiated, it can be seen that there is a water stream inside the pot. This raises the question to know if this only
oscillation could be sufficient to propel a boat.
 Three cutter stabs later... Three cutter stabs later...
    
Where we try to connect what is seen with the theory :
 This description/interpretation is based on the glass coil,
subject of this page. It is obvious that small discrepancies (time, speed, etc) could be expected/observed according to
the coil that is used. For the engines provided with a drum (with or without diaphragm) it seems there is no major difference
in the principle, but their geometry differs… therefore, if you think through this kind of engine, the vocabulary used here
might have to be adapted. This description/interpretation is based on the glass coil,
subject of this page. It is obvious that small discrepancies (time, speed, etc) could be expected/observed according to
the coil that is used. For the engines provided with a drum (with or without diaphragm) it seems there is no major difference
in the principle, but their geometry differs… therefore, if you think through this kind of engine, the vocabulary used here
might have to be adapted.
 In short, let's go!... In short, let's go!...
1)
Starting
 Pipe full. Heating. The first micro bubbles (a very small volume) correspond to the dissolved gas. The next ones are steam bubbles…
The vaporized water occupies more space than when it was liquid. The liquid water that is in the pipe is pushed… Pipe full. Heating. The first micro bubbles (a very small volume) correspond to the dissolved gas. The next ones are steam bubbles…
The vaporized water occupies more space than when it was liquid. The liquid water that is in the pipe is pushed…
 The pipe, in its air surrounded part, doesn't contain water any more, and then it fills again.
How can we interpret this observation ? The pipe, in its air surrounded part, doesn't contain water any more, and then it fills again.
How can we interpret this observation ?
 The volume occupied by the steam is (here) seen constant during about 30 seconds. Then water is sucked.
The water being sucked, necessarily it means that the pressure in the coil is less than when the steam volume was steady. The volume occupied by the steam is (here) seen constant during about 30 seconds. Then water is sucked.
The water being sucked, necessarily it means that the pressure in the coil is less than when the steam volume was steady.
 I recently read an assumption about steam overheating which would lead to a pressure decrease. Indeed, if we heat more and more
the steam, its pressure could decrease…but only if the volume it occupies increases (refer to the "perfect gas law": PV=nRT). Here, the volume occupied by
the steam while the pressure decrease occurs is obviously constant (even though some micro droplets of liquid water are suspended in the gas at the beginning
of the 30 second phase, their volume is negligible, and their vaporization doesn't lead to an additional volume). The hypothesis of steam overheating doesn't
allow to justify the pressure decrease that initiates the water sucking
(but finally I think Jean-Yves agrees on… and I probably badly interpreted some writings). I recently read an assumption about steam overheating which would lead to a pressure decrease. Indeed, if we heat more and more
the steam, its pressure could decrease…but only if the volume it occupies increases (refer to the "perfect gas law": PV=nRT). Here, the volume occupied by
the steam while the pressure decrease occurs is obviously constant (even though some micro droplets of liquid water are suspended in the gas at the beginning
of the 30 second phase, their volume is negligible, and their vaporization doesn't lead to an additional volume). The hypothesis of steam overheating doesn't
allow to justify the pressure decrease that initiates the water sucking
(but finally I think Jean-Yves agrees on… and I probably badly interpreted some writings).
 The only one explanation I could see (but I don't claim to be discovering it !...) is the one I have considered for a rather long time
without having put it in writing. It derived from the question: what happens at the liquid gas interface ? The only one explanation I could see (but I don't claim to be discovering it !...) is the one I have considered for a rather long time
without having put it in writing. It derived from the question: what happens at the liquid gas interface ?
 Let's start with some reminders of physics and chemistry. Let's take a liquid (considered as pure, in a sense that it contains only one type of molecules).
In equilibrium with this liquid it exists a vapor phase (=gas = dispersed molecules) made of the same molecules. This means that the molecules of the liquid, for instance water, move from liquid
to gas to join the other gasses forming the air. In the same time, some molecules of water as gas move to liquid. There is a "dynamic" equilibrium when as many molecules move in both directions. Let's start with some reminders of physics and chemistry. Let's take a liquid (considered as pure, in a sense that it contains only one type of molecules).
In equilibrium with this liquid it exists a vapor phase (=gas = dispersed molecules) made of the same molecules. This means that the molecules of the liquid, for instance water, move from liquid
to gas to join the other gasses forming the air. In the same time, some molecules of water as gas move to liquid. There is a "dynamic" equilibrium when as many molecules move in both directions.
 It is possible to shift this equilibrium, i.e. it is possible to have more molecules going in one direction than in the other one. To any temperature and pressure condition
corresponds an equilibrium. If the conditions are changed, the equilibrium status is replaced by a new one. It is possible to shift this equilibrium, i.e. it is possible to have more molecules going in one direction than in the other one. To any temperature and pressure condition
corresponds an equilibrium. If the conditions are changed, the equilibrium status is replaced by a new one.
 Let's come back to our coil. Let's come back to our coil.
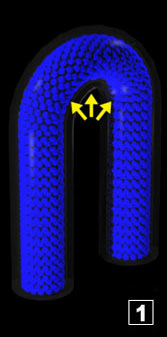
 The coil is filled with water. Inside there is only one phase. The coil is heated as well as the water that it contains. The water temperature increases until it reaches
the boiling temperature. At this moment, some water which was liquid starts to vaporize and creates a second phase (steam = gas). So long as some liquid water remains in the spirals (I insist, in the spirals),
the temperature doesn't increase above the boiling temperature. The status change occurs at constant temperature. The coil is filled with water. Inside there is only one phase. The coil is heated as well as the water that it contains. The water temperature increases until it reaches
the boiling temperature. At this moment, some water which was liquid starts to vaporize and creates a second phase (steam = gas). So long as some liquid water remains in the spirals (I insist, in the spirals),
the temperature doesn't increase above the boiling temperature. The status change occurs at constant temperature.
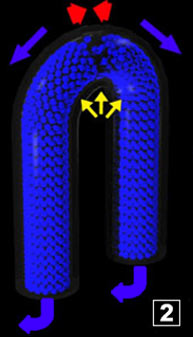
 This second phase (gas), for an equal quantity of molecules, takes more volume than the liquid water which originates it, which means it pushes
the liquid water remaining into the pipes. In addition, and it is important, as soon as the second phase exists there are molecule exchanges between liquid and gas. This second phase (gas), for an equal quantity of molecules, takes more volume than the liquid water which originates it, which means it pushes
the liquid water remaining into the pipes. In addition, and it is important, as soon as the second phase exists there are molecule exchanges between liquid and gas.
 When the interface has just been created, it is located in temperature conditions where the "equilibrium" is moved towards the steam generation (there is still a supply of energy).
The steam that is created continues to push water out of the pipes. When the interface has just been created, it is located in temperature conditions where the "equilibrium" is moved towards the steam generation (there is still a supply of energy).
The steam that is created continues to push water out of the pipes.
 Doing that, the interface climbs down in the pipes of which the temperature is less and less high when going away from the spirals (the flame heats much more the coil than
the rest of the exhaust pipes, and the bottom of the pipes is immerged in "cold" water). Thus, the equilibrium is less and less moving towards the steam generation when the interfaces goes away from the coil…
and finally we get a situation where the conditions are met for a reverse move, i.e. a re-condensation of the steam (as soon as the interface goes out of the zone where the temperature exceeds 100°C).
This occurs likely soon because the pipes can be touched with the fingers… Doing that, the interface climbs down in the pipes of which the temperature is less and less high when going away from the spirals (the flame heats much more the coil than
the rest of the exhaust pipes, and the bottom of the pipes is immerged in "cold" water). Thus, the equilibrium is less and less moving towards the steam generation when the interfaces goes away from the coil…
and finally we get a situation where the conditions are met for a reverse move, i.e. a re-condensation of the steam (as soon as the interface goes out of the zone where the temperature exceeds 100°C).
This occurs likely soon because the pipes can be touched with the fingers…
|
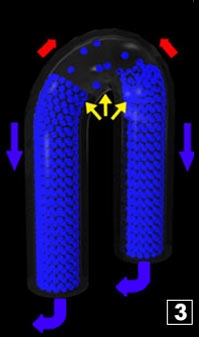
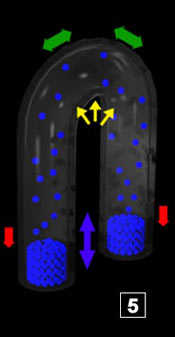
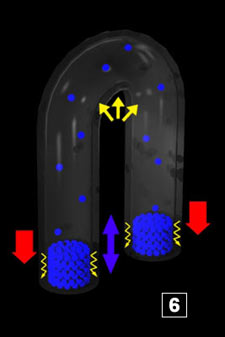
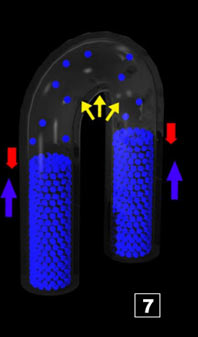
 Green arrows symbolize steam expansion when the temperature exceeds 100°C (locally at the top of the system). Double arrows symbolize situations where there is an equilibrium in the process. For the red "flow" = as many molecules cross the interface in a way and in the opposite way. For the green arrows = steam expansion stabilized (stopped). For liquid water = water doesn't move any more in the pipes. Green arrows symbolize steam expansion when the temperature exceeds 100°C (locally at the top of the system). Double arrows symbolize situations where there is an equilibrium in the process. For the red "flow" = as many molecules cross the interface in a way and in the opposite way. For the green arrows = steam expansion stabilized (stopped). For liquid water = water doesn't move any more in the pipes.
|
|
 However, though there is no more steam generation, the interface still moves
towards the pipe bottoms as a consequence of a likely certain expansion of the steam located inside the coil, the temperature
(and volume) of which being increasing (there is no more liquid water in the coil) until every point of the coil stabilises
("quickly") at a certain temperature, according to an equilibrium between the heat from the flame and the losses in the air ;
which would correspond to the situation where the interface becomes still. However, though there is no more steam generation, the interface still moves
towards the pipe bottoms as a consequence of a likely certain expansion of the steam located inside the coil, the temperature
(and volume) of which being increasing (there is no more liquid water in the coil) until every point of the coil stabilises
("quickly") at a certain temperature, according to an equilibrium between the heat from the flame and the losses in the air ;
which would correspond to the situation where the interface becomes still.
 Then, transiently there exists an "astable" equilibrium (there is a slow and invisible evolution
that looks like stability), i.e. some steam continues slowly to become liquid at the interface. Hence, the steam pressure
in the pipe decreases because the material quantity in steam phase decreases (refer to "perfect gas" equation PV=nRT, where
it is the decrease of n value that leads to the one of P and consequently of V). Now we come to a hypothesis which looks
plausible to justify a pressure decrease in the coil. Then, transiently there exists an "astable" equilibrium (there is a slow and invisible evolution
that looks like stability), i.e. some steam continues slowly to become liquid at the interface. Hence, the steam pressure
in the pipe decreases because the material quantity in steam phase decreases (refer to "perfect gas" equation PV=nRT, where
it is the decrease of n value that leads to the one of P and consequently of V). Now we come to a hypothesis which looks
plausible to justify a pressure decrease in the coil.
 However, everything is not yet clarified. Indeed, it can be seen the water is frankly re-sucked (it climbs up in the pipes with
a rather high speed and with a relatively long stroke (possibly varying, obviously). How could we justify this while the
low pressure generation is likely slow (in the steam phase the molecules are dispersed and the interface surface is small)
and it would make sense (subjective) to see the water being re-sucked once the low pressure is being created, i.e. relatively
slower than what can be seen. However, everything is not yet clarified. Indeed, it can be seen the water is frankly re-sucked (it climbs up in the pipes with
a rather high speed and with a relatively long stroke (possibly varying, obviously). How could we justify this while the
low pressure generation is likely slow (in the steam phase the molecules are dispersed and the interface surface is small)
and it would make sense (subjective) to see the water being re-sucked once the low pressure is being created, i.e. relatively
slower than what can be seen.
 Personally, I presume that the water is only re-sucked once the low pressure reaches a certain value, below which the sucking force is strong enough to cope with the gravity one
s (and the ones involved by the "wetting capacity" of water ?) which tend(s) to drag the water towards the bottom of the pipes. Personally, I presume that the water is only re-sucked once the low pressure reaches a certain value, below which the sucking force is strong enough to cope with the gravity one
s (and the ones involved by the "wetting capacity" of water ?) which tend(s) to drag the water towards the bottom of the pipes.
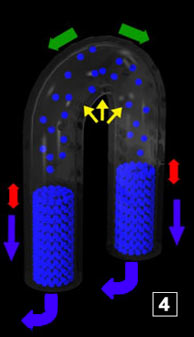
|  The yellow zig-zags represent the forces that more or less draw the water towards the bottom
of the pipes. Water climbs up as soon as enough steam has condensed so that the corresponding sucking force
is strong enough to cope with the resisting ones. Between 5 and 6, the material quantity (water) in the vapor phase
has decreased because of the re-condensing at the interface. From there comes the low pressure that originates the re-sucking
(the first one) of water (PV=nRT…). In 7, water is close to reach the area which is above 100°C, hence, flashing, etc… The yellow zig-zags represent the forces that more or less draw the water towards the bottom
of the pipes. Water climbs up as soon as enough steam has condensed so that the corresponding sucking force
is strong enough to cope with the resisting ones. Between 5 and 6, the material quantity (water) in the vapor phase
has decreased because of the re-condensing at the interface. From there comes the low pressure that originates the re-sucking
(the first one) of water (PV=nRT…). In 7, water is close to reach the area which is above 100°C, hence, flashing, etc…
|
|  Before considering what follows in the cycle, a couple of things can be looked
at… When the interface stabilizes (as height) in the pipes, there is P steam = P atmo (+ P water column, but it is negligible
compared to P atmo). One could like to know what is the liquid water volume corresponding to the steam amount. Two ways to proceed…
both being exactly equal if we think well ! You will use the one you prefer… Before considering what follows in the cycle, a couple of things can be looked
at… When the interface stabilizes (as height) in the pipes, there is P steam = P atmo (+ P water column, but it is negligible
compared to P atmo). One could like to know what is the liquid water volume corresponding to the steam amount. Two ways to proceed…
both being exactly equal if we think well ! You will use the one you prefer…
a) PV=nRT
|
with |
P = 1 (P drum = P atmo) we neglect P exerted by water column between surface and pipe ends
V = V max occupied by the steam (as visible) = 1.5 cm3 (i.e. 1.5x10-3 L) (case of the glass coil)
T = 100°C = 373K (likely slightly more, but well…)
R = perfect gas constant
n = material quantity as gas, in moles
|
 n= PV/RT= (1
x 1,5.10-3) / (8,2.10-2 x 373) = 4,9.10-5 mol H2O n= PV/RT= (1
x 1,5.10-3) / (8,2.10-2 x 373) = 4,9.10-5 mol H2O
 which, with M = m/n = 18g/mol, gives 8.8x10-4 g i.e. 0.88 mg ... which, with M = m/n = 18g/mol, gives 8.8x10-4 g i.e. 0.88 mg ...
b) Where we use molar volumes :
|
1 mole of water as liquid at 25°C = 18x10-3 L
1 mole of water as vapor at 100°C = 30.58 L |
 |
The volume ratio is 1700 |
 Which give us for 1.5 mL of steam : 1.5/1700 = 8.8x10-4g i.e. 0.88 mg (as I said…) Which give us for 1.5 mL of steam : 1.5/1700 = 8.8x10-4g i.e. 0.88 mg (as I said…)
 Don't forget that the result is approximate (T in fact >100°C). It only gives a rough idea… But it means
the quantity of water that is vaporized is very small… (approximately 0.06% of the volume occupied later by the steam). Don't forget that the result is approximate (T in fact >100°C). It only gives a rough idea… But it means
the quantity of water that is vaporized is very small… (approximately 0.06% of the volume occupied later by the steam).
 Hence, it can be seen that the heating power should be limited, which
is checked in practice, if not the amount of water that is vaporized is too much and steam escapes by the pipe ends,
involving jerky start… because we are still talking about starting… and now we are going to see what is next… ... Hence, it can be seen that the heating power should be limited, which
is checked in practice, if not the amount of water that is vaporized is too much and steam escapes by the pipe ends,
involving jerky start… because we are still talking about starting… and now we are going to see what is next… ...
2) Stabilised running :
 Some water is sucked… when reaching the very hot surface at the top of
the pipes it vaporizes quasi instantaneously, thus the pressure inside the coil increases suddenly,
the water in the pipes being then pushed again towards the pipe ends… Some water is sucked… when reaching the very hot surface at the top of
the pipes it vaporizes quasi instantaneously, thus the pressure inside the coil increases suddenly,
the water in the pipes being then pushed again towards the pipe ends…
 Water is then very quickly re-sucked, etc,… Here we are ! We come to a cruising speed… Water is then very quickly re-sucked, etc,… Here we are ! We come to a cruising speed…
 In this phase where everything works, we can raise the question: where does the
re-sucking comes from? Obviously, as for the first re-sucking, it is very likely that part of the "excess" of
steam which originated the water exhaust re-condenses at the interface, hence pressure decrease, etc… In this phase where everything works, we can raise the question: where does the
re-sucking comes from? Obviously, as for the first re-sucking, it is very likely that part of the "excess" of
steam which originated the water exhaust re-condenses at the interface, hence pressure decrease, etc…
 However, though the volume of water that flashed is likely smaller than the one vaporized during the first draining (see below), it seems that the re-condensation at the interface, which remains a relatively slow phenomenon, doesn't suffice to justify totally, after the starting phase, the very fast generation of low pressures able to make the water climbs up several times per second… However, though the volume of water that flashed is likely smaller than the one vaporized during the first draining (see below), it seems that the re-condensation at the interface, which remains a relatively slow phenomenon, doesn't suffice to justify totally, after the starting phase, the very fast generation of low pressures able to make the water climbs up several times per second…
 Concerning this fast movement,Jean-Yves R. Concerning this fast movement, Jean-Yves R. proposes an interesting assumption based on the kinetic energy… He feels that the kinetic energy got by the "water sausage" which moves in the pipe initiates, when the water goes down, a sucking force… as the pressure inside a syringe decreases when the piston is drawn… or, closer analogy with what happens in a "Humphrey pump" of which the working is really based on the kinetic energy got by the water pushed in a pipe by an explosion of a gas mixture… (Here, our "explosion" would be the flashing of liquid water becoming steam…). Concerning this fast movement,Jean-Yves R. Concerning this fast movement, Jean-Yves R. proposes an interesting assumption based on the kinetic energy… He feels that the kinetic energy got by the "water sausage" which moves in the pipe initiates, when the water goes down, a sucking force… as the pressure inside a syringe decreases when the piston is drawn… or, closer analogy with what happens in a "Humphrey pump" of which the working is really based on the kinetic energy got by the water pushed in a pipe by an explosion of a gas mixture… (Here, our "explosion" would be the flashing of liquid water becoming steam…).
 Note : We can wonder how much water vaporizes during each cycle. Two possible approaches. We can imagine 5 to 10% of the quantity of water
vaporized initialy. Considering that 0,9 mg are vaporized during the starting (see end of "1-Starting"),
10% gives something like 0,09 mg (i.e. 90 µg, i.e. less 1/10 th of mm3 !).
Note : We can wonder how much water vaporizes during each cycle. Two possible approaches. We can imagine 5 to 10% of the quantity of water
vaporized initialy. Considering that 0,9 mg are vaporized during the starting (see end of "1-Starting"),
10% gives something like 0,09 mg (i.e. 90 µg, i.e. less 1/10 th of mm3 !).
 Second approach : if we neglect the phenomena bound to the elasticity of steam
, we could consider that the volume corresponding to the stroke of the "water snake" (7 to 10 mm) is the result of the formation of steam during each cyle.
In this case, the steam volume that is generated is 0,05.10-3 L (radius of pipe = 1,125mm, stroke = 10mm).
We use the "steam at 100°C/water" volume ratio (= 1700) => quantity of water vaporized during each cycle and corresponding to
the volume described by the interface is 2,9.10-8 L or with a round-off 3.10-5 mL or 0,03 mg (or 0,03 mm3, or something like
3% of the water amount vaporized at the beginning, or 0,0018 % of the water that there was in the coil before starting.
In short, in both cases we find tiny volumes... Second approach : if we neglect the phenomena bound to the elasticity of steam
, we could consider that the volume corresponding to the stroke of the "water snake" (7 to 10 mm) is the result of the formation of steam during each cyle.
In this case, the steam volume that is generated is 0,05.10-3 L (radius of pipe = 1,125mm, stroke = 10mm).
We use the "steam at 100°C/water" volume ratio (= 1700) => quantity of water vaporized during each cycle and corresponding to
the volume described by the interface is 2,9.10-8 L or with a round-off 3.10-5 mL or 0,03 mg (or 0,03 mm3, or something like
3% of the water amount vaporized at the beginning, or 0,0018 % of the water that there was in the coil before starting.
In short, in both cases we find tiny volumes...
 But they are in good accordance with the pop-pop starting procedure of Slater Harisson. Indeed, after he had filled the drum, he drains it in order to just leave some drops
on the walls of the drum... And the engine works fine ! But they are in good accordance with the pop-pop starting procedure of Slater Harisson. Indeed, after he had filled the drum, he drains it in order to just leave some drops
on the walls of the drum... And the engine works fine !
3) Summing up :
 The coil is full of water. The coil is full of water.
 The water is heated. The water is heated.
 A very small amount of water becomes steam (0,06% here). A very small amount of water becomes steam (0,06% here).
 The remaining water is pushed down in the pipes. The remaining water is pushed down in the pipes.
 It happens a time where this draining out stops because the water that could become steam is no longer in such conditions (there is less than 100°C) and the steam already created doesn't expand more than it is because the temperature inside the coil is stabilized (equilibrium between heating and dissipation in surrounding air). It happens a time where this draining out stops because the water that could become steam is no longer in such conditions (there is less than 100°C) and the steam already created doesn't expand more than it is because the temperature inside the coil is stabilized (equilibrium between heating and dissipation in surrounding air).
 A part of the created steam re-condenses at the liquid/gas interface (which is now in conditions
allowing this)… The material quantity in gas phase decreases, and the pressure decreases accordingly (PV=nRT).
(Note : We are in an open system and then the decrease of pressure is immediately compensated by P atmo => P is a pseudo-constant
=> that's V that decreases). A part of the created steam re-condenses at the liquid/gas interface (which is now in conditions
allowing this)… The material quantity in gas phase decreases, and the pressure decreases accordingly (PV=nRT).
(Note : We are in an open system and then the decrease of pressure is immediately compensated by P atmo => P is a pseudo-constant
=> that's V that decreases).
 The low pressure thus created becomes (more or less quickly) low enough to overcome the forces that drag water towards the bottom of the pipes…
Therefore, water climbs up. The low pressure thus created becomes (more or less quickly) low enough to overcome the forces that drag water towards the bottom of the pipes…
Therefore, water climbs up.
 The water going up reaches a very hot section of the coil. The water going up reaches a very hot section of the coil.
 A small amount is suddenly vaporized… involving a fast increase of the pressure which pushes again the water in the pipes, with a high speed. A small amount is suddenly vaporized… involving a fast increase of the pressure which pushes again the water in the pipes, with a high speed.
 Re-condensation at the interface and (moreover?) kinetic energy of the water are
generating a sucking force (low pressure) which applies very soon on the exhausting water. Re-condensation at the interface and (moreover?) kinetic energy of the water are
generating a sucking force (low pressure) which applies very soon on the exhausting water.
 Water climbs up again, vaporizes again suddenly (estimation : approx. 3% of the quantity of steam
created durind the first drainage, or 0,0018 % of the quantity of water that there was in the coil before the start), etc. etc. Water climbs up again, vaporizes again suddenly (estimation : approx. 3% of the quantity of steam
created durind the first drainage, or 0,0018 % of the quantity of water that there was in the coil before the start), etc. etc.
Where we talk about a couple of additional observations :
 On one hand, if we heat too much, we already saw that with the brass pop-pop,
the working is jerking (irregular). Now we can see that when there is more heat (bigger flame) the stroke is more erratic,
with sometimes long strokes of the water in the pipe. On one hand, if we heat too much, we already saw that with the brass pop-pop,
the working is jerking (irregular). Now we can see that when there is more heat (bigger flame) the stroke is more erratic,
with sometimes long strokes of the water in the pipe.
 On another hand, it is (commonly?) accepted that the boat progresses because there is a dissymmetry between exhaust, which is a waterjet following the direction of the outlet pipe, and sucking which is water comes from any direction around the pipe end. This looks like a good explanation, other examined parameters looking as playing a negligible role (dissymetry of the hull, etc.). On another hand, it is (commonly?) accepted that the boat progresses because there is a dissymmetry between exhaust, which is a waterjet following the direction of the outlet pipe, and sucking which is water comes from any direction around the pipe end. This looks like a good explanation, other examined parameters looking as playing a negligible role (dissymetry of the hull, etc.).
 At last, just to see, we tried to plug one of the pipe ends. On the closed side, some water stays in the pipe up to where the coil starts. On the open side, a normal oscillation is observed. This is an incitation to build very simple working engines: a simple cylindrical room prolonged by a thinner pipe as outlet. At last, just to see, we tried to plug one of the pipe ends. On the closed side, some water stays in the pipe up to where the coil starts. On the open side, a normal oscillation is observed. This is an incitation to build very simple working engines: a simple cylindrical room prolonged by a thinner pipe as outlet.
Where we conclude carefully :
 For sure, these tests were performed with only one engine, but as it is true that all (or almost all?) the coils that were built run, we are allowed to think that the cycle of the process is always similar to the one we observed. For sure, these tests were performed with only one engine, but as it is true that all (or almost all?) the coils that were built run, we are allowed to think that the cycle of the process is always similar to the one we observed.
 It is as well obvious that I don't claim to solve here a mystery… I think that others should have likely already thought about it… Perhaps what I tell has already been written… but it has not been brought to my knowledge, and I only tried to think… Right? I hope so! … But I am not sure… and I remain receptive to any comment, critic or other. It is as well obvious that I don't claim to solve here a mystery… I think that others should have likely already thought about it… Perhaps what I tell has already been written… but it has not been brought to my knowledge, and I only tried to think… Right? I hope so! … But I am not sure… and I remain receptive to any comment, critic or other.
 Well, I don't know what to add… Ah yes! It remains a hell of a job to do: building a coil which is as efficient as possible (stroke increase in particular), Therefore,.. let go to your pipes and enjoy !! Well, I don't know what to add… Ah yes! It remains a hell of a job to do: building a coil which is as efficient as possible (stroke increase in particular), Therefore,.. let go to your pipes and enjoy !!
April 2003
Update 1 : March 2005
Update 2 : Dec. 2006
« Back to "Pop-pop"
|